Last Updated:
25th January 2025
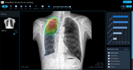
JF CXR-1 is an AI-powered screening and triaging tool to help clinicians identify abnormalities on chest X-rays. JF CXR-1 can also be used as a prioritization tool for radiologists and teleradiology companies.
Certification
China NMPA Class III
Development Stage
On the Market
Deployment
Online & Offline
Intended Age Group
15+ years
Target Setting
Primary health centres, teleradiology companies, government/public sector, e.g. national tuberculosis (TB) programme, private sector
Current Market
China
Input
Can be used to read images from any chest X-ray machine and model
Chest X-ray image format: DICOM
Chest X-ray type: Posterior-anterior chest X-ray, Anterior-posterior chest X-ray
Output
Output includes:
Heatmap
Dichotomous output indicating whether TB is likely present or absent
Dichotomous output indicating whether each abnormality is likely present or absent
Probability score for TB
Probability score for each abnormality
Location of each abnormality
The default cut-off probability score for TB is 0.35 and it cannot be adjusted.
JF CXR-1 returns disease scores. The subsequent products can format the results in a structured report.
Lung abnormalities included in the TB Score: Blunted costophrenic angle, Opacity, Pleural effusion
Additional findings reported by the product: Calcification, Fibrosis, Mass, Nodule, Opacity, Pleural effusion, Pneumothorax
Please note: There is limited independent evidence validating CAD for non-TB findings


Hardware
Minimum requirements:
Online mode: Intel Core i5, 8 GB memory, 500 GB disk space
Offline mode (local installation): Intel Core i5, 16 GB memory, 1 TB disk space
Server
All the leading VPS providers are supported.
Integration with X-ray Systems
Integration with PACS and Legacy Systems
We use DICOM 3.0 standard to receive DICOM images from X-ray systems.
We use DICOM 3.0 standard to receive DICOM images from PACS. We also provide a file uploader for the legacy data systems.
Software
A web browser is required to use JF CXR-1, e.g. viewing CXR, checking AI results. We recommend using a Chromium-based browser for the best experience.
Processing Time
It takes around 5 seconds per image with the minimum hardware requirements.
Data Sharing & Privacy
The software can be deployed locally and offline. There is an option to de-identify data.
Product Development Method
Supervised deep learning (CNN, RNN)
Training
The product was trained on over 120,000 chest X-rays from China
Reference Standard
Human reader
Publications
[1] X. Cao, Y. Li, H. Xin, H. Zhang, M. Pai, and L. Gao, “Application of artificial intelligence in digital chest radiography reading for pulmonary tuberculosis screening,” Chronic Diseases and Translational Medicine, vol. 7, no. 1, pp. 35–40, Mar. 2021, doi: 10.1016/j.cdtm.2021.02.001.
[2] Z. Z. Qin et al., “Tuberculosis detection from chest x-rays for triaging in a high tuberculosis-burden setting: an evaluation of five artificial intelligence algorithms,” The Lancet Digital Health, vol. 3, no. 9, pp. e543–e554, Sep. 2021, doi: 10.1016/S2589-7500(21)00116-3.
[3] Z. Z. Qin et al., “Computer-aided detection of tuberculosis from chest radiographs in a tuberculosis prevalence survey in South Africa: external validation and modelled impacts of commercially available artificial intelligence software,” The Lancet Digital Health, p. S2589750024001183, Jul. 2024, doi: 10.1016/S2589-7500(24)00118-3.
[4] Q. Liao et al., “Evaluation of an artificial intelligence (AI) system to detect tuberculosis on chest X-ray at a pilot active screening project in Guangdong, China in 2019,” XST, vol. 30, no. 2, pp. 221–230, Mar. 2022, doi: 10.3233/XST-211019.
[5] Y. Yang et al., “A prospective multicenter clinical research study validating the effectiveness and safety of a chest X-ray-based pulmonary tuberculosis screening software JF CXR-1 built on a convolutional neural network algorithm,” Front. Med., vol. 10, p. 1195451, Aug. 2023, doi: 10.3389/fmed.2023.1195451.
[6] A. J. Codlin et al., “Independent evaluation of 12 artificial intelligence solutions for the detection of tuberculosis,” Sci Rep, vol. 11, no. 1, p. 23895, Dec. 2021, doi: 10.1038/s41598-021-03265-0.
This website works best with browsers other than Internet Explorer.



